
Chemistry, 18.12.2019 04:31 broomssymphonie
There are three ways in which to define acids and bases: the arrhenius concept, the brønsted-lowry concept, and the lewis concept. arrhenius acids are substances that, when dissolved in water, increase the concentration of the h+ ion; arrhenius bases are substances that, when dissolved in water, increase the concentration of the oh− ion. brønsted-lowry acids are substances that can donate a proton (h+) to another substance; brønsted-lowry bases are substances that can accept a proton (h+). a lewis acid is an electron-pair acceptor, and a lewis base is an electron-pair donor. part a using the arrhenius concept of acids and bases, identify the arrhenius acid and base in each of the following reactions: 2koh(aq)+h2so4(aq)→k2so4(aq)+2h2o(l ) nh3(g)+hcl(g)→nh4cl(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1



Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
There are three ways in which to define acids and bases: the arrhenius concept, the brønsted-lowry...
Questions

Biology, 26.02.2021 01:40



Health, 26.02.2021 01:40

Mathematics, 26.02.2021 01:40

History, 26.02.2021 01:40

History, 26.02.2021 01:40

Biology, 26.02.2021 01:40


Chemistry, 26.02.2021 01:40

Chemistry, 26.02.2021 01:40

Mathematics, 26.02.2021 01:40


Mathematics, 26.02.2021 01:40




History, 26.02.2021 01:40


English, 26.02.2021 01:40

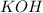 acts as base.
acts as base.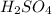 acts as acid.
acts as acid.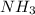 acts as base.
acts as base.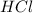 acts as acid.
acts as acid.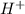 .
.
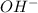 .
.
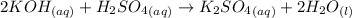
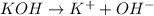 and hence, acts as base.
and hence, acts as base.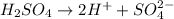 and hence, acts as acid.
and hence, acts as acid.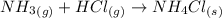
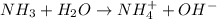 and hence, acts as base.
and hence, acts as base.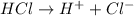 and hence, acts as acid.
and hence, acts as acid.

