Chemistry, 18.12.2019 04:31 angelvega2003
The great french chemist antoine lavoisier discovered the law of conservation of mass in part by doing a famous experiment in 1775. in this experiment lavoisier found that mercury(ii) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas.
1. write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(ii) oxide (hgo) into liquid mercury and gaseous dioxygen.
2. suppose 50.0ml of dioxygen gas are produced by this reaction, at a temperature of 90°c and pressure of exactly 1atm. calculate the mass of mercury(ii) oxide that must have reacted. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

You know the right answer?
The great french chemist antoine lavoisier discovered the law of conservation of mass in part by doi...
Questions

Mathematics, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50




Mathematics, 03.03.2021 19:50



Mathematics, 03.03.2021 19:50

Chemistry, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50





Mathematics, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50


Mathematics, 03.03.2021 19:50

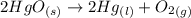
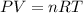
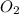 = 0.001677 mol
= 0.001677 mol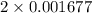 moles of mercury(II) oxide are reacted
moles of mercury(II) oxide are reacted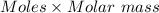 =
=