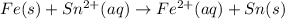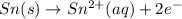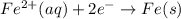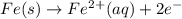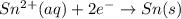What we call "tin cans" are really iron cans coated with a thin layer of tin. the anode is a bar of tin and the cathode is the iron can. an electrical current is used to oxidize the sn to sn2+ in solution, which is reduced to produce a thin coating of sn on the can.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
What we call "tin cans" are really iron cans coated with a thin layer of tin. the anode is a bar of...
Questions


Biology, 29.07.2019 11:10


Mathematics, 29.07.2019 11:10

Biology, 29.07.2019 11:10






Mathematics, 29.07.2019 11:10

Advanced Placement (AP), 29.07.2019 11:10


Mathematics, 29.07.2019 11:10