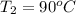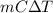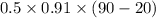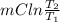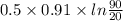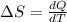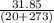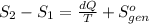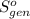Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. calculate (a) the change in stored energy (δe), (b) the amount of heat transfer (q), (c) the change in entropy (δs), (d) the amount of entropy transfer by heat and (e) the entropy generation (sgen, univ) in the system's universe during the heat transfer process.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. c...
Questions




Social Studies, 03.11.2020 18:20



Mathematics, 03.11.2020 18:20


History, 03.11.2020 18:20


History, 03.11.2020 18:20

Mathematics, 03.11.2020 18:20



Mathematics, 03.11.2020 18:20

Mathematics, 03.11.2020 18:20

Mathematics, 03.11.2020 18:20

Social Studies, 03.11.2020 18:20


Arts, 03.11.2020 18:20

 ,
,