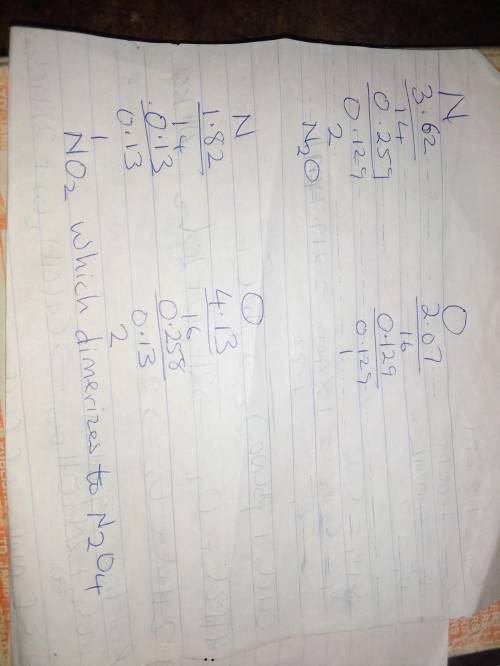Nitrogen and oxygen can react to form various compounds.
two experiments showed that one comp...

Chemistry, 18.12.2019 05:31 alishajade
Nitrogen and oxygen can react to form various compounds.
two experiments showed that one compound is formed when 3.62 g of nitrogen and 2.07 g of oxygen react completely, while another compound is formed when 1.82 g of nitrogen reacts completely with 4.13 g of oxygen.
which of the following are most likely the molecular formulas for the nitrogen oxides obtained in these experiments? (1) no, n2o(2) no, no2(3) n2o, n2o5(4) no, n2o4(5) n2o, n2o4

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Questions













History, 07.11.2019 03:31

Mathematics, 07.11.2019 03:31




Physics, 07.11.2019 03:31

Biology, 07.11.2019 03:31

Mathematics, 07.11.2019 03:31