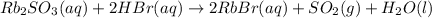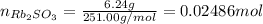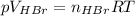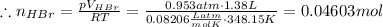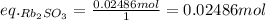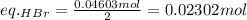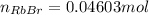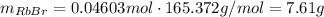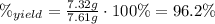Chemistry, 18.12.2019 07:31 Zachary429
Asolid sample of rb2so3 weighing 6.24 g reacts with 1.38 l gaseous hbr, measured at 75°c and 0.953 atm pressure. the solid rbbr, extracted from the reaction mixture and purified, has a mass of 7.32 g.
(a) what is the limiting reactant?
(b) what is the theoretical yield of rbbr, assuming com- plete reaction?
(c) what is the actual percentage yield of product?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1
You know the right answer?
Asolid sample of rb2so3 weighing 6.24 g reacts with 1.38 l gaseous hbr, measured at 75°c and 0.953 a...
Questions


Mathematics, 12.12.2019 19:31

Physics, 12.12.2019 19:31







Chemistry, 12.12.2019 19:31


History, 12.12.2019 19:31


History, 12.12.2019 19:31

Mathematics, 12.12.2019 19:31




Mathematics, 12.12.2019 19:31