
Chemistry, 18.12.2019 19:31 cyaransteenberg
At a given temperature, the elementary reaction a − ⇀ ↽ − b , in the forward direction, is first order in a with a rate constant of 0.0340 s − 1 . the reverse reaction is first order in b and the rate constant is 0.0740 s − 1 . what is the value of the equilibrium constant for the reaction a − ⇀ ↽ − b at this temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1
You know the right answer?
At a given temperature, the elementary reaction a − ⇀ ↽ − b , in the forward direction, is first ord...
Questions

History, 04.08.2019 07:30



Mathematics, 04.08.2019 07:30

History, 04.08.2019 07:30


Chemistry, 04.08.2019 07:30

Mathematics, 04.08.2019 07:30




Health, 04.08.2019 07:30

Social Studies, 04.08.2019 07:30

Biology, 04.08.2019 07:30

Physics, 04.08.2019 07:30

Health, 04.08.2019 07:30

Chemistry, 04.08.2019 07:30



Social Studies, 04.08.2019 07:30

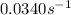
![R=K[A]](/tpl/images/0424/5632/3dc0d.png)
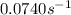
![R'=K'[B]](/tpl/images/0424/5632/2c128.png)
![K[A]=K'[B]](/tpl/images/0424/5632/af2e7.png)
![K_{eq}=\frac{[B]}{[A]}=\frac{K}{K'}=\frac{0.0340 s^{-1}}{0.0740 s^{-1}}=0.4595](/tpl/images/0424/5632/574c7.png)


