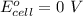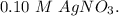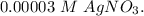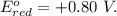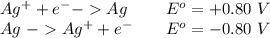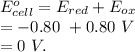Aconcentration cell is constructed by using the same half-reaction for both the cathode and anode. what is the cell potential e at 298k for a concentration cell that combines a silver cathode in contact with 0.10 m silver nitrate and a silver anode in contact with 0.3 m silver nitrate? (eored = +0.80 v for ag/ag+)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Aconcentration cell is constructed by using the same half-reaction for both the cathode and anode. w...
Questions

Mathematics, 07.07.2021 04:00

Mathematics, 07.07.2021 04:00





Computers and Technology, 07.07.2021 04:00





History, 07.07.2021 04:00

Mathematics, 07.07.2021 04:00


History, 07.07.2021 04:00




Mathematics, 07.07.2021 04:00

History, 07.07.2021 04:00