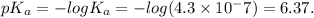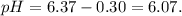Chemistry, 18.12.2019 20:31 12233445566
The ka of carbonic acid (h2co3) is 4.3 x 10–7. a solution of sodium hydrogen carbonate (nahco3) solution is created by dissolving 4.00 moles of sodium hydrogen carbonate in 2.00 l of aqueous solution. what is the ph of the solution at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
The ka of carbonic acid (h2co3) is 4.3 x 10–7. a solution of sodium hydrogen carbonate (nahco3) solu...
Questions

Chemistry, 31.08.2019 16:30


Mathematics, 31.08.2019 16:30


English, 31.08.2019 16:30


History, 31.08.2019 16:30

Biology, 31.08.2019 16:30


Business, 31.08.2019 16:30


Social Studies, 31.08.2019 16:30

Biology, 31.08.2019 16:30

Mathematics, 31.08.2019 16:30


Biology, 31.08.2019 16:30


History, 31.08.2019 16:30


History, 31.08.2019 16:30

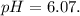
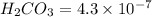 .
.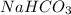 , M=
, M=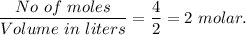
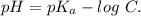 ...1
...1