
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxygen gas to produce iron (iii) oxide. your sample of iron is 12.0 moles of iron. so which if these is a true statement? note: all numbers located immediately after elemental symbols below should be considered subscripts. a. 4.5 moles of o2 and produce 3.0 moles of fe2o3. b. 12.0 moles of o2 and produce 24.0 moles of fe2o3. c. 9.0 moles of o2 and produce 3.0 moles of fe2o3. d. 9.0 moles of o2 and produce 6.0 moles of fe2o3 e. none of the above

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2
You know the right answer?
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxy...
Questions

History, 04.03.2020 05:20








Mathematics, 04.03.2020 05:20

Mathematics, 04.03.2020 05:20



Spanish, 04.03.2020 05:21

Computers and Technology, 04.03.2020 05:21



Social Studies, 04.03.2020 05:21

Business, 04.03.2020 05:21

Social Studies, 04.03.2020 05:22

Biology, 04.03.2020 05:22

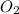 and produce 6.0 moles of
and produce 6.0 moles of 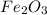
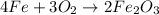
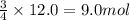 of oxygen gas
of oxygen gas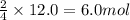 of iron (III) oxide
of iron (III) oxide

