Chemistry, 19.12.2019 00:31 dianaskye9081
The equilibrium constant for the reaction ni2+(aq) + 6 nh3(aq) ⇌ ni(nh3)6 2+(aq) is kf = 5.6 × 108 at 25°c. (a) what is δg o at this temperature? (b) if standard-state concentrations of reactants and products are mixed, in which direction does the reaction proceed? (c) determine δg when [ni(nh3)62+] = 0.010 m, [ni2+] = 0.0010 m, and [nh3] = 0.0050 m. in which direction will the reaction proceed to achieve equilibrium? (a) × 10 j/mol (enter your answer in scientific notation.) (b) to the right. to the left. (c) × 10 j/mol (enter your answer in scientific notation.) to the right. to the left.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3


Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
The equilibrium constant for the reaction ni2+(aq) + 6 nh3(aq) ⇌ ni(nh3)6 2+(aq) is kf = 5.6 × 108 a...
Questions










Computers and Technology, 28.11.2019 18:31

History, 28.11.2019 18:31

Mathematics, 28.11.2019 18:31




History, 28.11.2019 18:31




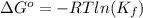
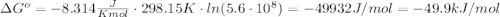
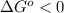 . This means reaction spontaneously proceeds to the right side. Besides, K > 1, this means products dominate over reactants, so reaction proceeds to the right.
. This means reaction spontaneously proceeds to the right side. Besides, K > 1, this means products dominate over reactants, so reaction proceeds to the right.![Q_f = \frac{[Ni(NH_3)_6]^{2+}}{[Ni^{2+}][NH_3]^6} = \frac{0.010}{0.0010\cdot 0.0050^6} = 6.4\cdot 10^{14}](/tpl/images/0425/0507/3ae25.png)
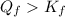 . In this case, we have an excess of the products, this means reaction will shift to the let left to restore the equilibrium.
. In this case, we have an excess of the products, this means reaction will shift to the let left to restore the equilibrium.