Chemistry, 19.12.2019 00:31 dgayles8761
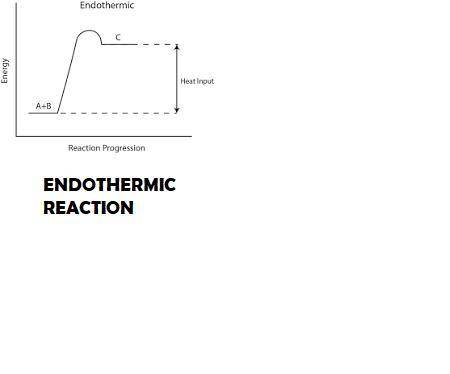
The dissolution of ammonium nitrate, nh4no3, in water is an endothermic process. since the calorimeter is not a perfect insulator, will the enthalpy of solution for ammonium nitrate be reported as too high or too low if this heat change is ignored? explain

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

You know the right answer?
The dissolution of ammonium nitrate, nh4no3, in water is an endothermic process. since the calorimet...
Questions

Mathematics, 05.12.2020 02:30

Mathematics, 05.12.2020 02:30

Social Studies, 05.12.2020 02:30





Geography, 05.12.2020 02:30



Mathematics, 05.12.2020 02:30

Mathematics, 05.12.2020 02:30





Mathematics, 05.12.2020 02:30


Mathematics, 05.12.2020 02:30

English, 05.12.2020 02:30