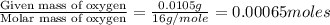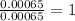Chemistry, 19.12.2019 00:31 janahiac09
A) combustion analysis of toluene, a common organic solvent, gives 5.86 mg of co2 and 1.37 mg of h2o. if the compound contains only carbon and hydrogen, what is its empirical formula?
b) menthol, the substance we can smell in mentholated cough drops, is composed of c, h, and o. a 0.1005-g sample of menthol is combusted, producing 0.2829 g of co2 and 0.1159 g of h2o. what is the empirical formula for menthol? if menthol has a molar mass of 156 g> mol, what is its molecular formula?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

You know the right answer?
A) combustion analysis of toluene, a common organic solvent, gives 5.86 mg of co2 and 1.37 mg of h2o...
Questions

Geography, 28.01.2020 08:31



Biology, 28.01.2020 08:31


Mathematics, 28.01.2020 08:31


Mathematics, 28.01.2020 08:31


Mathematics, 28.01.2020 08:31

History, 28.01.2020 08:31

English, 28.01.2020 08:31

English, 28.01.2020 08:31


Arts, 28.01.2020 08:31




History, 28.01.2020 08:31

History, 28.01.2020 08:31

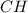

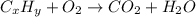

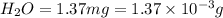
 of carbon dioxide,
of carbon dioxide,  of carbon will be contained.
of carbon will be contained. of water,
of water,  of hydrogen will be contained.
of hydrogen will be contained.

 moles.
moles.


 = 0.2829 g
= 0.2829 g = 0.1159 g
= 0.1159 g of carbon will be contained.
of carbon will be contained. of hydrogen will be contained.
of hydrogen will be contained.