
Chemistry, 19.12.2019 01:31 ayoismeisalex
The average bond energy (enthalpy) for a c=c double bond is 614 kj/mol and that of a c−c single bond is 348 kj/mol. estimate the energy needed to break only the π bond of the double bond of 2-butene. express your answer numerically in joules per molecule.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3


Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
The average bond energy (enthalpy) for a c=c double bond is 614 kj/mol and that of a c−c single bond...
Questions




Geography, 09.12.2020 17:20


Arts, 09.12.2020 17:20

Mathematics, 09.12.2020 17:20

English, 09.12.2020 17:20

Chemistry, 09.12.2020 17:20

Mathematics, 09.12.2020 17:20



Mathematics, 09.12.2020 17:20


Social Studies, 09.12.2020 17:20

Chemistry, 09.12.2020 17:20

Mathematics, 09.12.2020 17:20


Mathematics, 09.12.2020 17:20

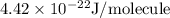 .
. 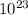
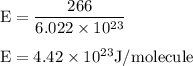
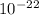 J/molecule.
J/molecule. 

