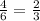Chemistry, 19.12.2019 01:31 asiababbie33
The al2o3 crystal structure (corundum) consists of an hcp arrangement of o2- ions; the al3+ ions occupy octahedral positions. what fraction of the available octahedral positions are filled with al3+ ions? in corundum, the al3+ ions do not fill the tetrahedral positions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
The al2o3 crystal structure (corundum) consists of an hcp arrangement of o2- ions; the al3+ ions oc...
Questions


Biology, 25.02.2021 20:40

Chemistry, 25.02.2021 20:40




Mathematics, 25.02.2021 20:40



Health, 25.02.2021 20:40

Chemistry, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40


Mathematics, 25.02.2021 20:40



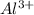 ions is
ions is 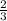
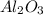 crystal structure consists of an HCP arrangement of
crystal structure consists of an HCP arrangement of 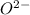 ions.
ions.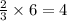
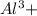 ions are present = 4
ions are present = 4