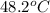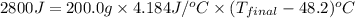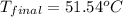Chemistry, 19.12.2019 02:31 mlarsen5000
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0 g solution at an initial temperature of 48.2°c. if the enthalpy of neutralization for the reaction between a strong acid and a strong base is −56 kj/mol, calculate the final temperature of the calorimeter contents. assume the specific heat capacity of the solution is 4.184 j°c⁻¹ g⁻¹ and assume no heat loss to the surroundings.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0...
Questions


Mathematics, 11.05.2021 20:10

History, 11.05.2021 20:10

Mathematics, 11.05.2021 20:10

Mathematics, 11.05.2021 20:10

Mathematics, 11.05.2021 20:20

Biology, 11.05.2021 20:20





Computers and Technology, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Law, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Chemistry, 11.05.2021 20:20

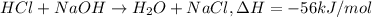
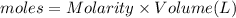
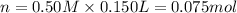
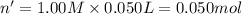
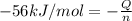
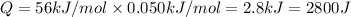
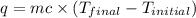
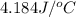
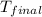 = final temperature =
= final temperature = 
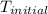 = initial temperature =
= initial temperature =