
Chemistry, 19.12.2019 04:31 hilarydodard7099
Refrigerant-134a enters the coils of the evaporator of a refrigeration system as a saturated liquid-vapor mixture at a pressure of 160 kpa. the refrigerant absorbs 180 kj/kg of heat from the cooled space, which is maintained at -5°c, and leaves as a saturated vapor at the same pressure. determine
(a) the entropy change of the refrigerant,
(b) the entropy change of the cooled space, and
(c) the total entropy change for this process.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

You know the right answer?
Refrigerant-134a enters the coils of the evaporator of a refrigeration system as a saturated liquid-...
Questions


Mathematics, 07.06.2020 18:57

Mathematics, 07.06.2020 18:57



English, 07.06.2020 18:57


English, 07.06.2020 18:57

Chemistry, 07.06.2020 18:57

Health, 07.06.2020 18:57

English, 07.06.2020 18:57




Mathematics, 07.06.2020 18:57


Mathematics, 07.06.2020 18:57

Business, 07.06.2020 18:57

Mathematics, 07.06.2020 18:57

Mathematics, 07.06.2020 19:57

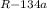 ) = Q/T
) = Q/T
 ) of the cooled space (ΔS
) of the cooled space (ΔS
 ) = ΔS
) = ΔS

