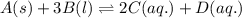Write the equilibrium‑constant expression for the reaction a ( s ) + 3 b ( l ) − ⇀ ↽ − 2 c ( aq ) + d ( aq ) in terms of [ a ] , [ b ] , [ c ] , and [ d ] , as needed. note that k c , which is sometimes symbolized as k eq , denotes that the equilibrium constant is expressed using molar concentrations. for this question, k c means the same thing as k eq .

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Write the equilibrium‑constant expression for the reaction a ( s ) + 3 b ( l ) − ⇀ ↽ − 2 c ( aq ) +...
Questions


Chemistry, 25.02.2022 20:10

Mathematics, 25.02.2022 20:10


English, 25.02.2022 20:20



History, 25.02.2022 20:20


Social Studies, 25.02.2022 20:20

Mathematics, 25.02.2022 20:20






Mathematics, 25.02.2022 20:20


History, 25.02.2022 20:20

![K_{eq}=[C]^2\times [D]](/tpl/images/0425/4433/d4d7d.png)
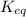
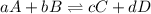
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0425/4433/9c8b0.png)