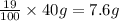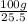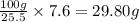A40.-g sample of impure kclo3 (solubility = 7.1 g per 100 g h2o at 20° c) is contaminated with 19 percent of kcl (solubility = 25.5 g per 100 g of h2o at 20° c). calculate the minimum quantity of 20° c water needed to dissolve all the kcl from the sample. (assume that the solubilities are unaffected by the presence of the other compound.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1


Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
A40.-g sample of impure kclo3 (solubility = 7.1 g per 100 g h2o at 20° c) is contaminated with 19 pe...
Questions



Mathematics, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Social Studies, 20.05.2021 01:00



History, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00



Mathematics, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

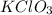 = 40 g
= 40 g