
Chemistry, 19.12.2019 20:31 harshakayla02
Ascientist measures the standard enthalpy change for the following reaction to be -190.0 kj : hcl(g) + nh3(g) nh4cl(s) based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of nh4cl(s) is kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

You know the right answer?
Ascientist measures the standard enthalpy change for the following reaction to be -190.0 kj : hcl(g...
Questions

Medicine, 18.09.2021 09:00

Geography, 18.09.2021 09:00

Business, 18.09.2021 09:00

History, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00


Chemistry, 18.09.2021 09:00

Social Studies, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00

Physics, 18.09.2021 09:00



Mathematics, 18.09.2021 09:00

Biology, 18.09.2021 09:00


Mathematics, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00

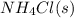 is -328.4 kJ/mol
is -328.4 kJ/mol![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(products)}]-\sum [n\times \Delta H_f_{(reactants)}]](/tpl/images/0426/4202/192b9.png)
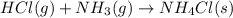
![\Delta H_{rxn}=[(1\times \Delta H_f_{(NH_4Cl(s))})]-[(1\times \Delta H_f_{(NH_3(g))})+(1\times \Delta H_f_{(HCl(g))})]](/tpl/images/0426/4202/589ac.png)
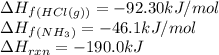
![-190.0=[(1\times \Delta H_f_{(NH_4Cl(s))})]-[(1\times (-46.1))+(1\times (-92.30))]\\\\\Delta H_f_{(NH_4Cl(s))}=-328.4kJ/mol](/tpl/images/0426/4202/9648b.png)


