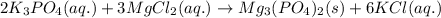Chemistry, 19.12.2019 20:31 raylynnreece5052
Aqueous potassium phosphate was mixed with aqueous magnesium chloride, and a crystallized magnesium phosphate product was formed. consider the other product and its phase, and then write the balanced molecular equation for this precipitation reaction. express your answer as a chemical equation including phases.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identify the balanced chemical equation that represents a decomposition reaction. p4 + 3o2 ⟶ p2o3 2fe(oh)3 ⟶ 2feo3 + h2o cuso4 ⟶ cuo + 2so3 2fe(oh)3 ⟶ fe2o3 + 3h2o
Answers: 1

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1
You know the right answer?
Aqueous potassium phosphate was mixed with aqueous magnesium chloride, and a crystallized magnesium...
Questions


Mathematics, 09.03.2021 22:30


Mathematics, 09.03.2021 22:30


Mathematics, 09.03.2021 22:30




Mathematics, 09.03.2021 22:30


Mathematics, 09.03.2021 22:30

Chemistry, 09.03.2021 22:30

Health, 09.03.2021 22:30




Mathematics, 09.03.2021 22:30


Advanced Placement (AP), 09.03.2021 22:30