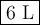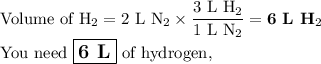n2 + 3h2 —> 2nh3
suppose you have 2.0 l of nitrogen. how many liters of hydrogen do you n...

Chemistry, 19.12.2019 22:31 astridantonio225
n2 + 3h2 —> 2nh3
suppose you have 2.0 l of nitrogen. how many liters of hydrogen do you need for a
complete reaction? (one mole of any gas occupies 22.4 l under certain conditions of
temperature and pressure. assume those conditions for this question.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

You know the right answer?
Questions

English, 03.07.2019 17:00




Mathematics, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00










History, 03.07.2019 17:00



Social Studies, 03.07.2019 17:00