Chemistry, 19.12.2019 22:31 mostman077
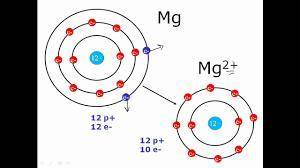
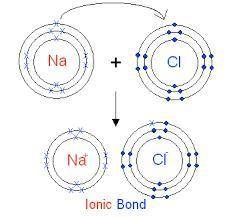
Elaborate on how the duet and octet rules relate to ion formation?
a) the duet rule or the octet rule state that atoms form ions in an effort to acquire valence electron configuration impermanence.
b) both the duet and octet rules reveal that atoms gain or lose electrons forming ions to achieve a more stable electron configuration.
c) ion formation only occur if the atom have a full duet electron configuration or full octet electron configuration of valence electrons.
d) formation of immutable noble-gas configuration ions results from atoms with ambiguous duet or octet configurations of valence electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2


Chemistry, 23.06.2019 13:30
Which factors would have influenced earth’s climate during the time of pangea? check all that apply. land covered by glaciers one large landmass one large ocean tectonic activity multiple small seas
Answers: 2
You know the right answer?
Elaborate on how the duet and octet rules relate to ion formation?
a) the duet rule or the o...
a) the duet rule or the o...
Questions


Mathematics, 10.10.2019 00:30



English, 10.10.2019 00:30


Mathematics, 10.10.2019 00:30


Mathematics, 10.10.2019 00:30

English, 10.10.2019 00:30



English, 10.10.2019 00:30

History, 10.10.2019 00:30

Chemistry, 10.10.2019 00:30




Mathematics, 10.10.2019 00:30

History, 10.10.2019 00:30