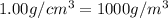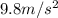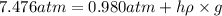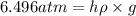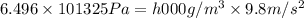Chemistry, 19.12.2019 23:31 hosteenimport21
The volume of a bubble that starts at the bottom of a lake at 4.55°c increases by a factor of 8.00 as it rises to the surface where the temperature is 18.05°c and the air pressure is 0.980 atm. assuming that the density of the lake water is 1.00 g/cm3, determine the depth of the lake?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
The volume of a bubble that starts at the bottom of a lake at 4.55°c increases by a factor of 8.00 a...
Questions

Advanced Placement (AP), 23.08.2019 23:50

Health, 23.08.2019 23:50

Chemistry, 23.08.2019 23:50



Mathematics, 23.08.2019 23:50

Biology, 23.08.2019 23:50

Mathematics, 23.08.2019 23:50



Mathematics, 23.08.2019 23:50


Mathematics, 23.08.2019 23:50


History, 23.08.2019 23:50

Mathematics, 23.08.2019 23:50

Mathematics, 23.08.2019 23:50


Mathematics, 23.08.2019 23:50

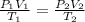
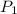 = initial pressure of gas in bubble= ?
= initial pressure of gas in bubble= ?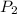 = final pressure of gas = 0.980 atm
= final pressure of gas = 0.980 atm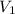 = initial volume of gas =
= initial volume of gas = 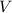
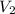 = final volume of gas = 8.00 × V
= final volume of gas = 8.00 × V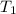 = initial temperature of gas =
= initial temperature of gas = 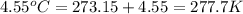
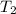 = final temperature of gas =
= final temperature of gas = 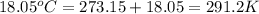
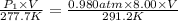
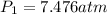
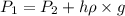
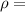 density of water =
density of water =