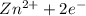Chemistry, 20.12.2019 00:31 adantrujillo1234
Consider an oxygen-concentration cell consisting of two zinc electrodes. one is immersed in a water solution with a low oxygen concentration and the other in a water solution with a high oxygen concentration. the zinc electrodes are connected by an external copper wire. (a) which electrode will corrode? (b) write half-cell reactions for the anodic reaction and the cathodic reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Consider an oxygen-concentration cell consisting of two zinc electrodes. one is immersed in a water...
Questions

Mathematics, 13.04.2021 04:00


Mathematics, 13.04.2021 04:00

Social Studies, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00





Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00



Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00

Mathematics, 13.04.2021 04:00

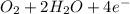 ⇒
⇒ 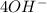
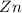 ⇒
⇒