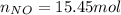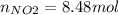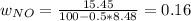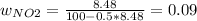Chemistry, 20.12.2019 00:31 ashleypere99
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reactions come to equilibrium after combustion in an internal-combustion engine at 2000 k and 200 bar, estimate the mole fractions of no and no2 present for mole fractions of nitrogen and oxygen in the combustion products of 0.70 and 0.05.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reaction...
Questions

Mathematics, 06.04.2021 17:20

Mathematics, 06.04.2021 17:20


Mathematics, 06.04.2021 17:20

Geography, 06.04.2021 17:20

Biology, 06.04.2021 17:20

Arts, 06.04.2021 17:20



Mathematics, 06.04.2021 17:20

Mathematics, 06.04.2021 17:20




English, 06.04.2021 17:20



Mathematics, 06.04.2021 17:20


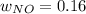
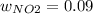
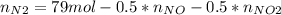
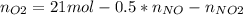
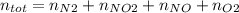
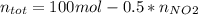
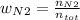
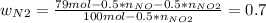
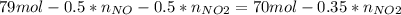
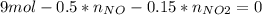 (1)
(1)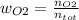
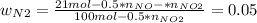
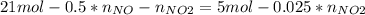
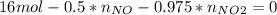 (2)
(2)