
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd(s) 2 ag(s) + cd2+ (a) voltage increases. (b) voltage decreases but remains > zero. (c) voltage becomes zero and remains at zero. (d) no change in voltage occurs. (e) direction of voltage change cannot be predicted without additional information. which of the above occurs for each of the following circumstances? 14. a 50-milliliter sample of a 2-molar cd(no3)2 solution is added to the left beaker. 15. the silver electrode is made larger. 16. the salt bridge is replaced by a platinum wire. 17. current is allowed to flow for 5 minutes.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd...
Questions

Mathematics, 25.08.2021 19:20


Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20



Chemistry, 25.08.2021 19:20

Computers and Technology, 25.08.2021 19:20



Mathematics, 25.08.2021 19:20

History, 25.08.2021 19:20



Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

History, 25.08.2021 19:20

English, 25.08.2021 19:20

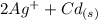 ⇒
⇒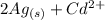
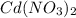 solution is added to the left beaker.
solution is added to the left beaker. 

