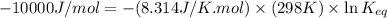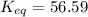Chemistry, 20.12.2019 01:31 maelaysiap
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than does oxygen (o2), as indicated by these approximate standard free-energy changes in blood:
reaction a: reaction b: hb+o2hb+co⟶⟶hbo2,hbco, δg∘=−70 kj/mol δg∘=−80 kj/mol
estimate the equilibrium constant k at 298 k for the equilibrium
hbo2+co⇌hbco+o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than doe...
Questions

English, 12.01.2021 18:40

Mathematics, 12.01.2021 18:40



Arts, 12.01.2021 18:40


Computers and Technology, 12.01.2021 18:40

Mathematics, 12.01.2021 18:40

Mathematics, 12.01.2021 18:40


Computers and Technology, 12.01.2021 18:40

Mathematics, 12.01.2021 18:40

History, 12.01.2021 18:40

History, 12.01.2021 18:40

Mathematics, 12.01.2021 18:40


Mathematics, 12.01.2021 18:40



Physics, 12.01.2021 18:40

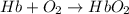 ;
; 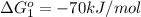
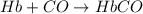 ;
; 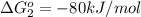
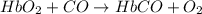 ;
; 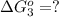
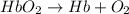 ;
; 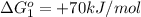
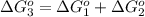
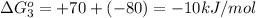
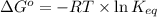
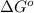 = standard Gibbs free energy = -10kJ/mol = -10000 J/mol
= standard Gibbs free energy = -10kJ/mol = -10000 J/mol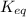 = equilibrium constant = ?
= equilibrium constant = ?