
Chemistry, 20.12.2019 02:31 ameera1973
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calcium hydroxide are added to this system, indicate whether the following statements are true or false. (assume that the volume does not change upon the addition of calcium hydroxide.)
a. the number of moles of ch3cooh will increase.
b. the number of moles of ch3coo- will decrease.
c. the equilibrium concentration of h3o will remain the same.
d. the ph will decrease.
e. the ratio of [ch3cooh] / [ch3coo-] will increase.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2


Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calc...
Questions



Mathematics, 23.10.2020 02:01

English, 23.10.2020 02:01


French, 23.10.2020 02:01

Spanish, 23.10.2020 02:01

Biology, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01


English, 23.10.2020 02:01


Advanced Placement (AP), 23.10.2020 02:01


Mathematics, 23.10.2020 02:01

Chemistry, 23.10.2020 02:01

Advanced Placement (AP), 23.10.2020 02:01


Mathematics, 23.10.2020 02:01

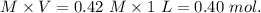
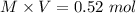
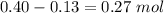
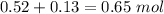
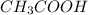 decrease and
decrease and 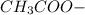 increase.
increase.

