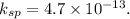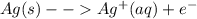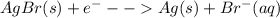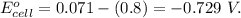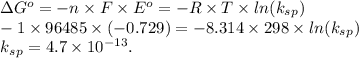Ag+(aq) + e- → ag(s) e° = +0.800 v agbr(s) + e- → ag(s) + br-(aq) e° = +0.071 v br2(l) + 2 e- → 2 br-(aq) e° = +1.066 v use some of the data above to calculate ksp at 25°c for agbr. enter your answer in exponential format (sample 1.23e-4) with two decimal places and no units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Ag+(aq) + e- → ag(s) e° = +0.800 v agbr(s) + e- → ag(s) + br-(aq) e° = +0.071 v br2(l) + 2 e- → 2 br...
Questions




Biology, 20.09.2019 10:30

Mathematics, 20.09.2019 10:30

English, 20.09.2019 10:30

Physics, 20.09.2019 10:30





History, 20.09.2019 10:30




Biology, 20.09.2019 10:30


Mathematics, 20.09.2019 10:30


Mathematics, 20.09.2019 10:30