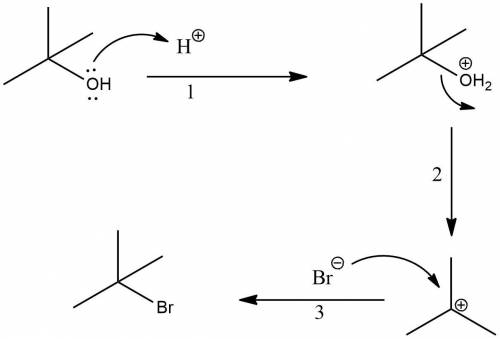
Chemistry, 20.12.2019 03:31 brutalgitaffe
Butyl bromide (2-brom-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking it with an aqueous solution of hbr at room temperature. the reaction is much faster than with n-butyl alcohol and is essentially 100% complete within a few minutes. give a mechanism for this reaction. note: it is not the same mechanism as for the lab preparation of 1-bromobutane. what is this reaction called?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
Butyl bromide (2-brom-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking...
Questions


Physics, 25.01.2022 02:10




Mathematics, 25.01.2022 02:10


Mathematics, 25.01.2022 02:10


Mathematics, 25.01.2022 02:10



Mathematics, 25.01.2022 02:10


Business, 25.01.2022 02:10




Mathematics, 25.01.2022 02:10

English, 25.01.2022 02:10

 (substitution nucleophilic bimolecular) reaction
(substitution nucleophilic bimolecular) reaction .
.