
Asystem undergoes a two-step process. in step 1, it absorbs 50. j of heat at constant volume. in step 2, it releases 5j of heat at 1 atm. as it returned to its original internal energy. find the change in the volume of the system during the second step and identify it as an expansion or compression.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
Asystem undergoes a two-step process. in step 1, it absorbs 50. j of heat at constant volume. in ste...
Questions

English, 03.10.2019 03:30

Chemistry, 03.10.2019 03:30

Chemistry, 03.10.2019 03:30


Mathematics, 03.10.2019 03:30

Spanish, 03.10.2019 03:30



English, 03.10.2019 03:30

Mathematics, 03.10.2019 03:30

English, 03.10.2019 03:30

History, 03.10.2019 03:30


History, 03.10.2019 03:30

Health, 03.10.2019 03:30





Mathematics, 03.10.2019 03:30

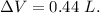
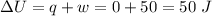
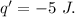
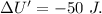
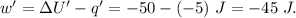
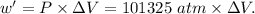
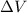 is positive.
is positive.

