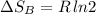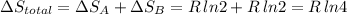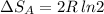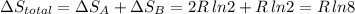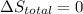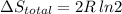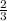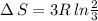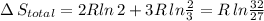Chemistry, 20.12.2019 04:31 blakesmith0110
Arigid container is divided into two compartments of equal volume by a partition. one compartment contains 1 mole of ideal gas a at 1 atm, and the other contains 1 mole of ideal gas b at 1 atm. calculate the increase in entropy which occurs when the partition between the two compartments is removed. if the frst compartment had contained 2 moles of ideal gas a, what would have been the increase in entropy when the partition was removed? calculate the corresponding increases in entropy in each of the preceding two situations if both compartments had contained ideal gas a.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2



Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Arigid container is divided into two compartments of equal volume by a partition. one compartment co...
Questions

Business, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01


Biology, 23.06.2020 23:01


Medicine, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01



Mathematics, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01

English, 23.06.2020 23:01


Mathematics, 23.06.2020 23:01

Mathematics, 23.06.2020 23:01



Mathematics, 23.06.2020 23:01

History, 23.06.2020 23:01

 .
.