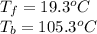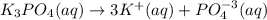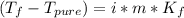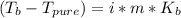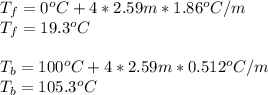Chemistry, 26.10.2019 21:43 therealnana
Assuming 100% dissociation, calculate the freezing point and boiling point of 2.59 m k3po4(aq).

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Assuming 100% dissociation, calculate the freezing point and boiling point of 2.59 m k3po4(aq)....
Questions

Mathematics, 06.11.2020 22:20


Biology, 06.11.2020 22:20

Social Studies, 06.11.2020 22:20

Social Studies, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20



Mathematics, 06.11.2020 22:20



Mathematics, 06.11.2020 22:20

Computers and Technology, 06.11.2020 22:20


English, 06.11.2020 22:20


Geography, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20