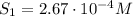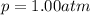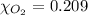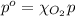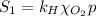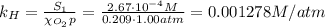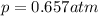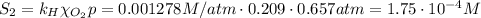The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the solubility of o2 from the air is 2.67 ✕ 10-4 m at sea level and 25°c, what is the solubility of o2 at an elevation of 12,000 ft where the atmospheric pressure is 0.657 atm? assume the temperature is 25°c, and that the mole fraction of o2 in air is 0.209 at both 12,000 ft and at sea level.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the so...
Questions

English, 22.11.2020 15:50

Mathematics, 22.11.2020 15:50


World Languages, 22.11.2020 15:50







Business, 22.11.2020 16:00

Chemistry, 22.11.2020 16:00

Business, 22.11.2020 16:00





History, 22.11.2020 16:00

History, 22.11.2020 16:10

Chemistry, 22.11.2020 16:10

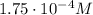
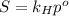
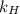 is Henry's law constant.
is Henry's law constant.