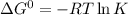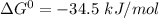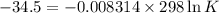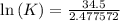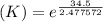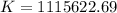Chemistry, 20.12.2019 18:31 alexabbarker9781
Determine the equilibrium constant for the following reaction at 298 k.
cl(g) + o3(g) → clo(g) + o2(g)
δg° = - 34.5 kj

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
You know the right answer?
Determine the equilibrium constant for the following reaction at 298 k.
cl(g) + o3(g) → clo(g)...
cl(g) + o3(g) → clo(g)...
Questions


Mathematics, 02.12.2020 05:40

History, 02.12.2020 05:40


History, 02.12.2020 05:40

Mathematics, 02.12.2020 05:40


Mathematics, 02.12.2020 05:40

Computers and Technology, 02.12.2020 05:40

Mathematics, 02.12.2020 05:40


Mathematics, 02.12.2020 05:40

English, 02.12.2020 05:40

Mathematics, 02.12.2020 05:40

Mathematics, 02.12.2020 05:40

Geography, 02.12.2020 05:40


Mathematics, 02.12.2020 05:40

Law, 02.12.2020 05:40

History, 02.12.2020 05:40