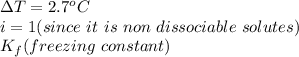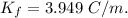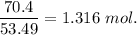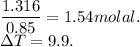Chemistry, 20.12.2019 19:31 savyblue1724707
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezing point of the solution is 2.7°c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh4ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x.
a) calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezi...
Questions

Social Studies, 19.10.2020 23:01




Computers and Technology, 19.10.2020 23:01


Computers and Technology, 19.10.2020 23:01

Computers and Technology, 19.10.2020 23:01






Mathematics, 19.10.2020 23:01


Engineering, 19.10.2020 23:01


English, 19.10.2020 23:01


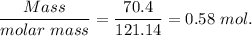
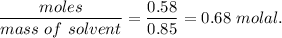
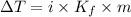 .....1
.....1