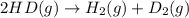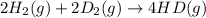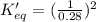Chemistry, 20.12.2019 19:31 kayleedavis08
The equilibrium constant is given for one of the reactions below.
determine the value of the missing equilibrium constant.
2 hd(g) ⇌ h2(g) + d2(g) kc = 0.28
2 h2(g) + 2 d2(g) ⇌ 4 hd(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
The equilibrium constant is given for one of the reactions below.
determine the value o...
determine the value o...
Questions

Mathematics, 03.08.2019 20:00


Biology, 03.08.2019 20:00


Arts, 03.08.2019 20:00


Mathematics, 03.08.2019 20:00

Social Studies, 03.08.2019 20:00

Mathematics, 03.08.2019 20:00

Mathematics, 03.08.2019 20:00

Biology, 03.08.2019 20:00

Mathematics, 03.08.2019 20:00

Arts, 03.08.2019 20:00

Mathematics, 03.08.2019 20:00


Mathematics, 03.08.2019 20:00



Chemistry, 03.08.2019 20:00