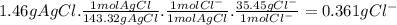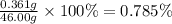To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.1600 m silver nitrate (agno3) solution to a 46.00 g sample of the fluid and collects the solid silver chloride (agcl) product. when no more agcl is produced, he filters, washes and weighs it, and finds that 1.46 g has been produced.
the balanced chemical equation for the reaction is:
cl^- (aq) + agno3(aq) > agcl(s) + no3^- (aq)
1. what kind of reaction is this?
o precipitation o acid-base o redox
2. if you said this was a precipitation reaction, enter the chemical formula of the precipitate.
3. if you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base.
4. if you said this was a redox reaction, enter the chemical symbol of the element that is oxidized.
5. calculate the mass percent of cl in the sample. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1
You know the right answer?
To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.1600 m silver...
Questions

Social Studies, 02.08.2019 07:30

Geography, 02.08.2019 07:30


Mathematics, 02.08.2019 07:30

Biology, 02.08.2019 07:30


Mathematics, 02.08.2019 07:30

Mathematics, 02.08.2019 07:30

Geography, 02.08.2019 07:30





Mathematics, 02.08.2019 07:30

History, 02.08.2019 07:30


Health, 02.08.2019 07:30



English, 02.08.2019 07:30