Chemistry, 20.12.2019 20:31 wrightstephanie193
Agalvanic (voltaic) cell consists of an electrode composed of titanium in a 1.0 m titanium(ii) ion solution and a second electrode composed of tin in a 1.0 m tin(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of titanium in a 1.0 m titanium(ii) ion s...
Questions



Mathematics, 25.06.2019 04:00


Mathematics, 25.06.2019 04:00



Mathematics, 25.06.2019 04:00




Mathematics, 25.06.2019 04:00

Mathematics, 25.06.2019 04:00







![E^0_{[Sn^{2+}/Sn]}=-0.14V](/tpl/images/0428/1023/81a51.png)
![E^0_{[Ti^{2+}/Ti]}=-1.63V](/tpl/images/0428/1023/456c4.png)
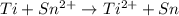
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Ti^{2+}]}{[Sn^{2+}]}](/tpl/images/0428/1023/a72a7.png)
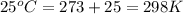
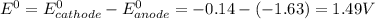
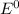 are standard reduction potentials.
are standard reduction potentials.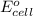 = standard electrode potential of the cell = 1.49 V
= standard electrode potential of the cell = 1.49 V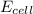 = emf of the cell = ?
= emf of the cell = ?