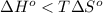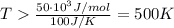Chemistry, 20.12.2019 20:31 mokunola16
At what temperature would a given reaction become spontaneous if h = kj and s = j/k? the instructor will choose a value for delta h between 50 kj and 250 kj and delta s between 100 j/k and 300 j/k. the correct answer must show the relationship between delta h and delta s that is used to calculate the temperature at which the change occurs as well as the calculations themselves. the final answer must have the correct units with it. any answer that simply shows a numerical value with units will not be accepted.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
At what temperature would a given reaction become spontaneous if h = kj and s = j/k? the instruct...
Questions

Chemistry, 04.12.2019 07:31

Mathematics, 04.12.2019 07:31

History, 04.12.2019 07:31

Mathematics, 04.12.2019 07:31




Mathematics, 04.12.2019 07:31

Mathematics, 04.12.2019 07:31

History, 04.12.2019 07:31


Social Studies, 04.12.2019 07:31






Mathematics, 04.12.2019 07:31

English, 04.12.2019 07:31


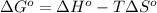 ;if the change in the Gibbs free energy is negative, the reaction is spontaneous, that is:
;if the change in the Gibbs free energy is negative, the reaction is spontaneous, that is: 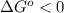 or
or 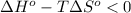 ;if the change in the Gibbs free energy is positive, the reaction is non-spontaneous, that is:
;if the change in the Gibbs free energy is positive, the reaction is non-spontaneous, that is: 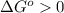 or
or 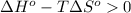 .
.