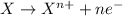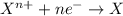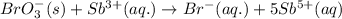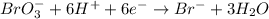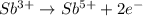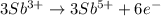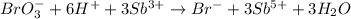Chemistry, 20.12.2019 21:31 holaadios222lol
Complete and balance the following redox equation using the set of smallest whole-number coefficients. now sum the coefficients of all species in the balanced equation. (remember the coefficients that are equal to one.) bro3-(aq) + sb3+(aq) ? br-(aq) + sb5+(aq) (acid solution) the sum of the coefficients is

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Complete and balance the following redox equation using the set of smallest whole-number coefficient...
Questions

Mathematics, 24.04.2020 21:32

English, 24.04.2020 21:32



Social Studies, 24.04.2020 21:32






Chemistry, 24.04.2020 21:32

Biology, 24.04.2020 21:32

Social Studies, 24.04.2020 21:32


Mathematics, 24.04.2020 21:32

Mathematics, 24.04.2020 21:32

Social Studies, 24.04.2020 21:32

Mathematics, 24.04.2020 21:32


World Languages, 24.04.2020 21:32