
Chemistry, 20.12.2019 23:31 michaelchavez6959127
In a different experiment, a student places a piece of pure ba(s) in a beaker containing 250.ml of 6.44mhcl(aq) and observes that the rate of appearance of h2(g) bubbles when ba(s) was added is much faster than the rate of appearance of bubbles when the mg(s) was added. the student claims that the greater reactivity is due to the difference in ionization energy between mg and ba. explain the difference in ionization energy between mg and ba in terms of atomic structure.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
In a different experiment, a student places a piece of pure ba(s) in a beaker containing 250.ml of 6...
Questions




Biology, 05.11.2019 15:31




Biology, 05.11.2019 15:31

Physics, 05.11.2019 15:31

History, 05.11.2019 15:31

Mathematics, 05.11.2019 15:31


Chemistry, 05.11.2019 15:31

Health, 05.11.2019 15:31



History, 05.11.2019 15:31


Health, 05.11.2019 15:31

Mathematics, 05.11.2019 15:31

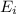
![[Ne]3s^2](/tpl/images/0428/3026/d3dcb.png)
![[Xe]6s^2](/tpl/images/0428/3026/f6f70.png)


