
Chemistry, 21.12.2019 02:31 leeahnnfoster
Anabolic steroids are sometimes used illegally by athletes to increase muscle strength. a forensic chemist analyzes some tablets suspected of being a popular steroid.
he determines the substance contains only c, h and o and has a molar mass of 300.42g/mol.
when a 1.200g sample is subjected to combustion analysis, 3.516g of co2 and 1.007g h2o are collected. a) what is the empirical formula of the unknown substance in the tablets? b)what is the molecular formula of the unknown substance in the tablets?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Anabolic steroids are sometimes used illegally by athletes to increase muscle strength. a forensic c...
Questions



Biology, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01





Geography, 20.10.2020 02:01

Biology, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01


History, 20.10.2020 02:01

History, 20.10.2020 02:01




Computers and Technology, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01

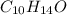
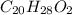
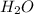 = 1.007 g /18 g/mol = 0.0559 moles
= 1.007 g /18 g/mol = 0.0559 moles
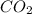 = 3.516 g /44.01 g/mol = 0.0799 moles
= 3.516 g /44.01 g/mol = 0.0799 moles


