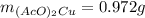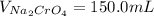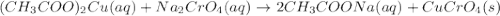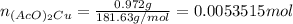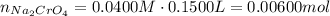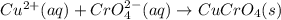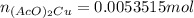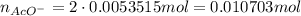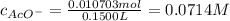Chemistry, 21.12.2019 05:31 nataliemoore1974
Suppose of copper(ii) acetate is dissolved in of a aqueous solution of sodium chromate. calculate the final molarity of acetate anion in the solution. you can assume the volume of the solution doesn't change when the copper(ii) acetate is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
Suppose of copper(ii) acetate is dissolved in of a aqueous solution of sodium chromate. calculate th...
Questions




Mathematics, 01.12.2021 03:10

English, 01.12.2021 03:10





English, 01.12.2021 03:10






SAT, 01.12.2021 03:10