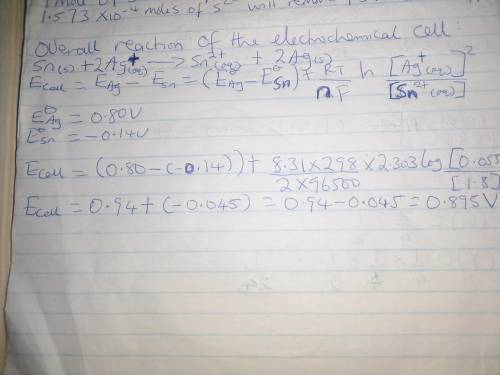Chemistry, 21.12.2019 06:31 koranbutterton
Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°c.
sn(s) sn2+(aq, 1.8 m) ag+(aq, 0.055 m) ag(s)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
Calculate the cell potential for the following reaction that takes place in an electrochemical cell...
Questions

Mathematics, 28.07.2019 13:30


Mathematics, 28.07.2019 13:30



Mathematics, 28.07.2019 13:30


Mathematics, 28.07.2019 13:30

English, 28.07.2019 13:30






English, 28.07.2019 13:30

Business, 28.07.2019 13:30


Biology, 28.07.2019 13:30


English, 28.07.2019 13:30