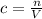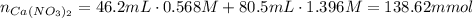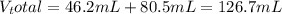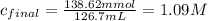Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
A46.2 ml,0.568 m calcium nitrate solution is mixed with 80.5ml of 1.396m calcium nitrate solution. c...
Questions

Mathematics, 27.01.2021 21:40



Mathematics, 27.01.2021 21:40



English, 27.01.2021 21:40

Business, 27.01.2021 21:40





Mathematics, 27.01.2021 21:40

Social Studies, 27.01.2021 21:40






Mathematics, 27.01.2021 21:40