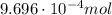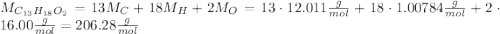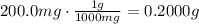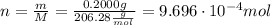Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
ibuprofen (c13h1802) is the active ingredient in many nonprescription pain relievers. each tablet co...
Questions

Biology, 27.10.2019 23:43



English, 27.10.2019 23:43


Mathematics, 27.10.2019 23:43

Mathematics, 27.10.2019 23:43



History, 27.10.2019 23:43





Mathematics, 27.10.2019 23:43

English, 27.10.2019 23:43

Mathematics, 27.10.2019 23:43

Chemistry, 27.10.2019 23:43

Biology, 27.10.2019 23:43

Mathematics, 27.10.2019 23:43