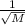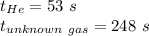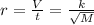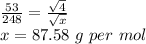Chemistry, 23.12.2019 18:31 22MadisonT
Asample of he gas (2.0 mmol) effused through a pinhole in 53 s. the same amount of an unknown gas, under the same conditions, effused through the pinhole in 248 s. the molecular mass of the unknown gas is g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1


Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2
You know the right answer?
Asample of he gas (2.0 mmol) effused through a pinhole in 53 s. the same amount of an unknown gas, u...
Questions

Biology, 25.09.2020 22:01



Mathematics, 25.09.2020 22:01

Arts, 25.09.2020 22:01



Arts, 25.09.2020 22:01

English, 25.09.2020 22:01






History, 25.09.2020 22:01


History, 25.09.2020 22:01

Mathematics, 25.09.2020 22:01

Mathematics, 25.09.2020 22:01

Biology, 25.09.2020 22:01