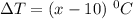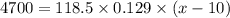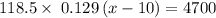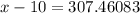Chemistry, 23.12.2019 18:31 someone2301
118.5 g piece of lead is heated with 4,700 j of energy. if the specific heat of lead is 0.129 j/ (g ⋅ °c), and the lead’s initial temperature was 10 °c, what is the final temperature of the lead?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3
You know the right answer?
118.5 g piece of lead is heated with 4,700 j of energy. if the specific heat of lead is 0.129 j/ (g...
Questions

English, 05.05.2021 22:00

Chemistry, 05.05.2021 22:00


Mathematics, 05.05.2021 22:00

Advanced Placement (AP), 05.05.2021 22:00


History, 05.05.2021 22:00

Chemistry, 05.05.2021 22:00




Mathematics, 05.05.2021 22:00

Social Studies, 05.05.2021 22:00

Mathematics, 05.05.2021 22:00




Mathematics, 05.05.2021 22:00

Mathematics, 05.05.2021 22:00

Mathematics, 05.05.2021 22:00

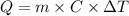
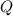 is the heat absorbed/released
is the heat absorbed/released is the temperature change
is the temperature change